Cement Mineralogy
Cement Mineralogy
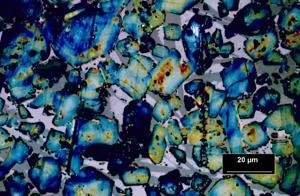
Hydraulic cements are our most important building materials. More than 2 billion t/a Ordinary Portland Cement (OPC) are produced world wide. Most of this material is consumed by concrete production and building chemistry formulations. Both are complex systems made from inorganic cement components, aggregates and organic additives. The organic additives yield the ability to minimize the amount of used cements which are produced by a huge input of energy (energy saving and minimization of CO2 emission). The picture above shows the thin section of an OPC clinker. By etching of the surface the clinker minerals are clearly to distinguish. The physical properties of the hardened cement are strongly influenced by the mineralogical composition (phase composition). The interaction of inorganic cement phases and organic molecules are in focus of our investigations. The early hydration behaviour of cementitous formulations are investigated by in-situ XRD analysis. The evolution of the phase content – starting from the clinker phases forming nano scaled hydration products – is recorded time dependend and is evaluated quantitatively. The linking of these results with the data of the calorimetric investigations are the basis of the development of new models of the interaction of organic additives and inorganic cement phases. The characterization of the solid cement stone allows statements with respect to its nano crystalline framework and its directly linked physical properties.
Prof. Dr. F. Goetz-Neunhoeffer, Prof. Dr. J. Neubauer, Dr. rer. nat. D. Jansen